List of work packages
Deliverables are developed within the following workpackages (WP), whose relationship is described in the Pert chart in figure 1.
| WP | Title | Lead beneficiary |
| 1 | Development of wearable physiological sensors | PLUX |
| 2 | Affective signal processing through the integration of multiple sources | UU |
| 3 | Affective classification, PlusMe AI and IM-TWIN integration | CNR |
| 4 | Validation of the PlusMe and IM-TWIN system | LA SAPIENZA |
| 5 | Exploitation of IM-TWIN system | CNR |
| 6 | Management and dissemination | CNR |
| 7 | Ethics requirements | CNR |
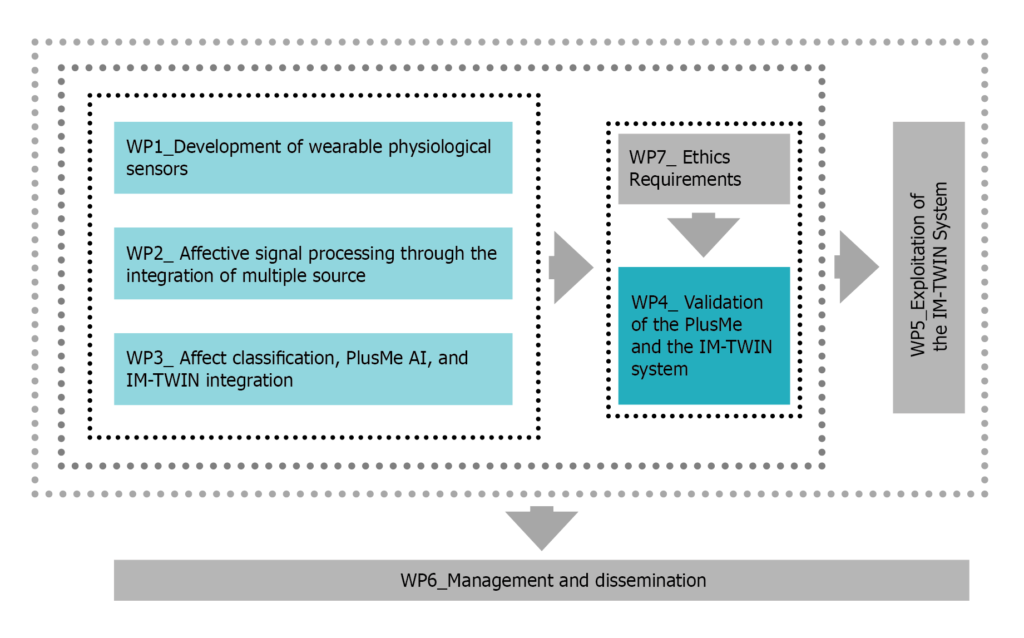
Figure 1: Pert chart describing the relation among the workpackages.
List of time-ordered deliverables
Deliverables are reported in the following time-ordered table. The Gantt chart in figure 2 shows the deliverables (and tasks too) against time, in the context of the respective workpackages.
| WP | No | Title | Lead beneficiary |
Nature | Dissemination Level | Due date | Due month |
| WP7 | D7.3 | POPD - Requirement No. 3 | CNR | Ethics | Confidential | 30 Nov 2020 | 1 |
| WP5 | D5.3 | IM-TWIN system Booklet 1 | CNR | Report | Public | 31 Jan 2021 | 3 |
| WP6 | D6.2 | Professional video on Project | CNR | Websites, patents filing, etc. | Public | 31 Jan 2021 | 3 |
| WP6 | D6.14 | Project web-site, Facebook, Twitter | CNR | Websites, patents filing, etc. | Public | 31 Jan 2021 | 3 |
| WP6 | D6.15 | Press KIT and press releases | CNR | Websites, patents filing, etc. | Public | 31 Jan 2021 | 3 |
| WP6 | D6.17 | Data Pilot report 1 | CNR | ORDP: Open Research Data Pilot | Public | 31 Jan 2021 | 3 |
| WP7 | D7.1 | POPD - Requirement No. 1 | CNR | Ethics | Confidential | 28 Feb 2021 | 4 |
| WP7 | D7.2 | POPD - Requirement No. 2 | CNR | Ethics | Confidential | 28 Feb 2021 | 4 |
| WP5 | 5.7 | Identification of target groups and relevant stakeholders 1 | CNR | Report | Confidential | 30 Apr 2021 | 6 |
| WP7 | D7.4 | H - Requirement No. 4 | CNR | Ethics | Confidential | 30 Apr 2021 | 6 |
| WP7 | D7.5 | POPD - Requirement No. 5 | CNR | Ethics | Confidential | 30 Apr 2021 | 6 |
| WP1 | D1.1 | Wearable Technical Design Description | PLUX | Report | Confidential | 31 Aug 2021 | 10 |
| WP1 | D1.2 | API software document | PLUX | Other | Public | 31 Oct 2021 | 12 |
| WP5 | D5.9 | IPR analysis and strategy 1 | CNR | Report | Confidential | 31 Jan 2022 | 15 |
| WP5 | D5.1 | Exploitation plan 2 | CNR | Report | Public | 28 Feb 2022 | 16 |
| WP3 | D3.3 | PlusMe AI-augmented behaviour, and IM-TWIN 1 | CNR | Demonstrator | Public | 28 Feb 2022 | 16 |
| WP3 | D3.5 | PlusMe production 1 | CNR | Demonstrator | Public | 31 Mar 2022 | 17 |
| WP1 | D1.3 | Physiological Wearable Sensors | PLUX | Demonstrator | Public | 31 May 2022 | 19 |
| WP3 | D3.6 | PlusMe production 2 | CNR | Demonstrator | Public | 31 Jul 2022 | 21 |
| WP5 | D5.5 | End-user engagement questionnaire 1 | CNR | Report | Confidential | 31 Jul 2022 | 21 |
| WP6 | D6.18 | Data Pilot report 2 | CNR | ORDP: Open Research Data Pilot | Public | 31 Jul 2022 | 21 |
| WP5 | D5.4 | IM-TWIN system Booklet 2 | CNR | Report | Public | 31 Aug 2022 | 22 |
| WP4 | D4.1 | Empirical validation: PlusMe | LA SAPIENZA | Report | Public | 31 Oct 2022 | 24 |
| WP5 | D5.14 | Draft Business Plan | CNR | Report | Confidential | 31 Oct 2022 | 24 |
| WP2 | D2.1 | Processing of physiological signals, visual information, and PlusMe interaction: first version | UU | Other | Public | 31 Dec 2022 | 26 |
| WP3 | D3.1 | Affect and emotional classification 1 | UU | Other | Public | 31 Dec 2022 | 26 |
| WP6 | D6.8 | Workshop for therapists and rehabilitation centers 1 | LA SAPIENZA | Other | Public | 31 Dec 2022 | 26 |
| WP3 | D3.7 | IM-TWIN production | CNR | Demonstrator | Public | 31 Jan 2023 | 27 |
| WP5 | D5.8 | Identification of target groups and relevant stakeholders 2 | CNR | Report | Confidential | 31 Jan 2023 | 27 |
| WP6 | D6.6 | Scientific workshop 1 | CNR | Other | Public | 31 Jan 2023 | 27 |
| WP5 | D5.11 | SWOT analysis, addressable-markets analysis | CNR | Report | Confidential | 28 Feb 2023 | 28 |
| WP6 | D6.5 | Professional video on IM-TWIN as a therapeutic tool | CNR | Websites, patents filing, etc. | Public | 31 Mar 2023 | 29 |
| WP5 | D5.12 | Country-based exploitation questionnaire and stakeholder interviews | CNR | Report | Confidential | 30 Apr 2023 | 30 |
| WP6 | D6.12 | B2B Meeting 1 | PLUX | Other | Public | 31 Apr 2023 | 30 |
| WP3 | D3.2 | Personalized affect classification and feedback | UU | Other | Public | 31 May 2023 | 31 |
| WP5 | D5.6 | End-user engagement questionnaire 2 | CNR | Report | Confidential | 30 Jun 2023 | 32 |
| WP2 | D2.2 | Processing of physiological signals, visual information, and PlusMe interaction: last version | UU | Other | Public | 31 Jul 2023 | 33 |
| WP3 | D3.4 | PlusMe AI-augmented behaviour, and IM-TWIN 2 | CNR | Demonstrator | Public | 31 Jul 2023 | 33 |
| WP5 | D5.10 | IPR analysis and strategy 2 | CNR | Report | Confidential | 31 Jul 2023 | 33 |
| WP6 | D6.3 | Professional video on Project 2 | CNR | Websites, patents filing, etc. | Public | 31 Aug 2023 | 34 |
| WP5 | D5.2 | Exploitation plan 3 | CNR | Report | Public | 30 Sep 2023 | 35 |
| WP6 | D6.9 | Workshop for therapists and rehabilitation centers 2 | CRI | Other | Public | 30 Sep 2023 | 35 |
| WP6 | D6.19 | Data Pilot report 3 | CNR | ORDP: Open Research Data Pilot | Public | 30 Sep 2023 | 35 |
| WP6 | D6.7 | Scientific workshop 2 | CNR | Other | Public | 31 Oct 2023 | 36 |
| WP6 | D6.10 | Open-day for families 1 | CRI | Other | Public | 31 Oct 2023 | 36 |
| WP6 | D6.11 | Open-day for families 2 | LA SAPIENZA | Other | Public | 31 Oct 2023 | 36 |
| WP6 | D6.13 | B2B Meeting 2 | UU | Other | Public | 31 Oct 2023 | 36 |
| WP6 | D6.16 | Press Conference | CNR | Websites, patents filing, etc. | Public | 31 Oct 2023 | 36 |
| WP5 | D5.13 | Feasibility study and business model | CNR | Report | Confidential | 31 Oct 2023 | 36 |
| WP4 | D4.2 | Empirical validation: IM-TWIN | LA SAPIENZA | Report | Public | 31 Oct 2023 | 36 |
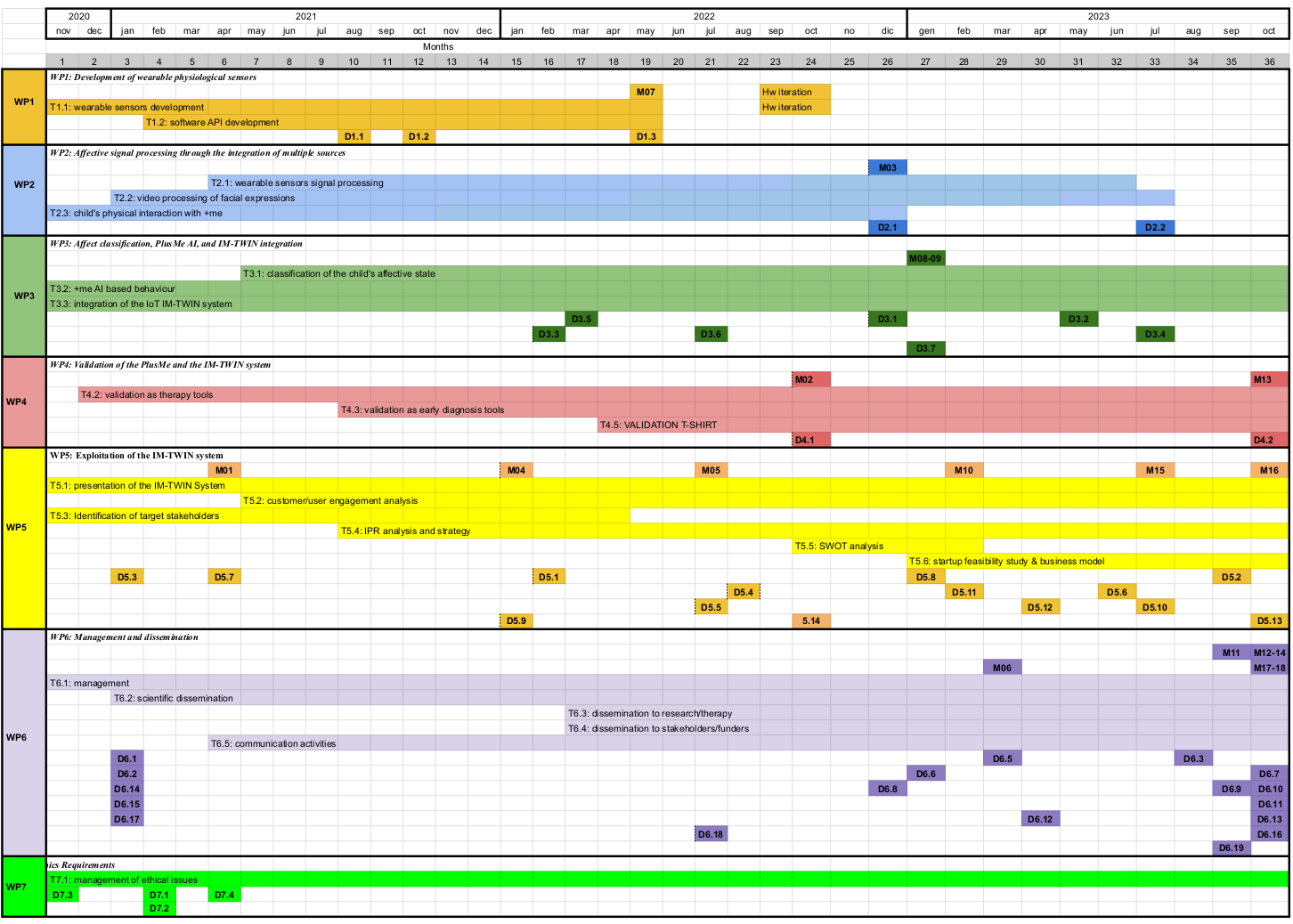
Figure 2: Gantt chart describing the timetable of deliverables, tasks and milestones.
